New Cancer after Transplant or CAR T-cell Therapy: Who's at Risk?
New Cancers After Transplant or CAR T-cell Therapy: Who's at Risk.
Monday, May 5, 2025
Presenter: Marcelo Pasquini MD, MS Medical College of Wisconsin, CIBMTR
Presentation is 31 minutes with 25 minutes of Q & A
Summary: Stem cell or bone marrow transplantation and CAR T-cell therapy can increase the risk of second cancers. This presentation describes the types and frequency of such risks along with strategies to mitigate them.
Key Points:
- Second cancers after transplant can be associated with infection, chemotherapy, and genetic predispositions. The more intense the pre-transplant chemotherapy, the greater the risk of second cancers.
- The risk of second cancers after CAR T-cell therapy is relatively low (less than 1% to 6.4%) and varies depending on the type of second cancer.
- Risk assessment, active cancer screening and annual visits can reduce the risk of second cancers after transplantation as well as CAR T-cell therapy.
(02:13): A ‘new cancer’ is not a relapse or recurrence of an original cancer.
(03:57): Second cancers can be a cancer in the same spectrum of diseases as the initial cancer.
(05:08): Complex treatment histories can make it difficult to determine if the second cancer was caused by transplant or CAR T-cell therapy, or something else.
07:24): The likelihood of developing a cancer diagnosis increases with age.
(12:07): The risk of disease relapse is highest early after transplant.
(13:04): The timeframe when patients typically develop a new cancer differs by disease.
(18:03): Overall survival after autologous and allogeneic transplants has improved over the last two decades.
(19:28): Second cancers can occur after transplant, although the risk is low. Cancer education and prevention are part of all survivorship guidelines for patient care.
(26:11): The most common malignancies reported to CIBMTR after CAR T-cell therapy were skin malignancies and myelodysplastic syndrome (MDS).
(30:11): The benefits of CAR T-cell therapy outweigh the risks.
Transcript of Presentation.
(00:01) Steve Buechler: Introduction. Good afternoon and welcome to the workshop New Cancers After Transplant or CAR T-cell Therapy: Who's at Risk. My name is Steve Buechler, and I will be your moderator for this workshop.
(00:11): It's my pleasure to introduce today's speaker, Dr. Marcelo Pasquini. Dr. Pasquini is a Professor of Medicine in Hematology and Oncology, and a member of the Blood and Marrow Transplant Group at the Medical College of Wisconsin in Milwaukee. His clinical work focuses on transplantation and cellular therapies, such as CAR T-cell therapy, to treat different diseases such as multiple myeloma, acute leukemia, myelofibrosis, and myelodysplastic syndrome. Please join me in welcoming Dr. Pasquini.
(00:41): Dr. Marcelo Pasquini: Overview of Talk. Hello. Thank you very much Steve, and it's a pleasure to be here to participate in this symposium. I will, for the next 30 minutes, talk about the complications of post-transplant and CAR T-cell therapy, mostly related with the development of new cancers.
(01:02): Here are my disclosures. This is related to my work with CAR T-cell therapy and the outcomes of CAR T-cell with the transplant registry.
(01:24): Today I will cover the following topics: The development of new cancers, with their definitions and considerations, followed by a few examples. I will illustrate what we mean about this and the main risk related to this complication.
(01:42): We first need to understand the components and risk factors for developing these malignancies after a bone marrow transplant or a CAR T-cell therapy. In terms of CAR T-cell, this is a fairly new topic. Although similar to a transplant, there are some peculiarities that I'd like to cover in relation to questions regarding key risk factors - whom, why, when, and how.
(02:13): A ‘new cancer’ is not a relapse or recurrence of an original cancer. So what are new cancers? It must be emphasized that a new cancer is not a relapse, or recurrence, of the original cancer diagnosis. Rather, it's the development of a new malignancy that is not directly related to the malignancy that the transplant was initially used to treat.
(02:33): Here are a few terms you will see in the literature about this. One of them is subsequent primary neo-plasms (SPN), a favored term especially for survivorship studies. The other term is subsequent primary malignancy (SPM). They are abbreviated as SPN or SPM.
(02:55): We try to avoid using the term secondary cancer because that implies that they were related to prior treatment. Oftentimes, one does not know the relationship, or causality, of these events.
(03:07): Another term that is used frequently is therapy-related malignancy, such as therapy-related acute myeloid leukemia, or therapy-related myelodysplastic syndrome.
(03:22): Subsequent cancers may happen after any cancer therapy and also in the absence of cancer therapy. So, patients with skin cancer may develop other cancers in the future in the absence of specific treatment, aside from surgical treatment, for that skin cancer.
(03:44): After CAR T-cell therapy, one important message to convey is that despite the risk, the benefits of the treatment outweigh the risk of this complication. I will now go over this in a little bit more detail.
(03:57): Second cancers can be a cancer in the same spectrum as the initial cancer. For example, ductal carcinoma inside the breast can transform into an invasive breast cancer.
(04:16): Some events are pre-cancers, for example MGUS (monoclonal gammopathy of uncertain significance) a detectable protein in the blood that can eventually increase the risk for developing symptomatic multiple myeloma.
(04:34): Similarly, MDS (myelodysplastic syndrome) was also considered a pre-leukemic state in the past, because patients tend to have a higher risk of developing acute myeloid leukemia after this diagnosis.
(04:46): Another example is patients who develop chronic lymphocytic leukemia, which can transform into large cell lymphoma. All of these are examples of diseases that are cancers which fall in the same trajectory of development as the primary one that was initially detected.
(05:08): Complex treatment histories can make it difficult to determine if the second cancer was caused by transplant or CAR T-cell therapy, or something else. We then have other complex relationships. For example, a multiple myeloma patient who received an autologous transplant, could possibly develop myelodysplastic syndrome (MDS). This would require an allogeneic transplant to treat the MDS. This, in turn, could lead to graft-versus-host disease (GVHD), which could possibly develop into basal cell skin cancer. This example shows that there could be three distinct different cancers in the patient's trajectory of treatments.
(05:43): Another example is of patients receiving breast cancer treatment with chemotherapy and radiation and then developing myelodysplastic syndrome (MDS) as a result of exposure to these therapies. Subsequently, this MDS could progress into acute leukemia, a disease in the same spectrum. Patients could then have an allogeneic transplant, further developing another illness called post-transplant lymphoproliferative disorder (PTLD) associated with an Epstein-Barr virus infection. These are complex situations that can occur in real life in real settings, with many of these examples being second malignancies.
(06:30): Another type of trajectory that has an unclear relationship is that of a patient who first develops a skin cancer called malignant melanoma, followed by another cancer, called multiple myeloma, which is unrelated to the melanoma. The patient then undergoes an autologous transplant for the multiple myeloma with lenalidomide (Revlimid®) maintenance as part of their therapy, but eventually, the melanoma returns. So, it's unclear whether the multiple myeloma is related to the melanoma, or whether the multiple myeloma, or its treatment, increased the chance for the relapse of melanoma. To reiterate, these are complex relationships, and with no full clarity whether the cancer, or the treatment, increase the risk for subsequent malignancy.
(07:24): Another important element is to understand is that the likelihood of developing a cancer diagnosis increases with age. As our organs age, there is an increased chance of accumulating errors that may result in the development of a malignancy. As can be seen in the SEER database, a US-wide epidemiologic assessment, an increase in age further increases the risk of any cancer.
(08:05): Looking specifically at myelodysplastic syndrome (MDS), there is a very steep increase in MDS cases after the age of 60. We now know that over time, people develop different predisposing factors—different clones in the bone marrow—that can eventually develop into MDS, and this risk increases with age. That tells you that as the bone marrow ages, errors may accumulate over time, increasing the chance for these diagnoses.
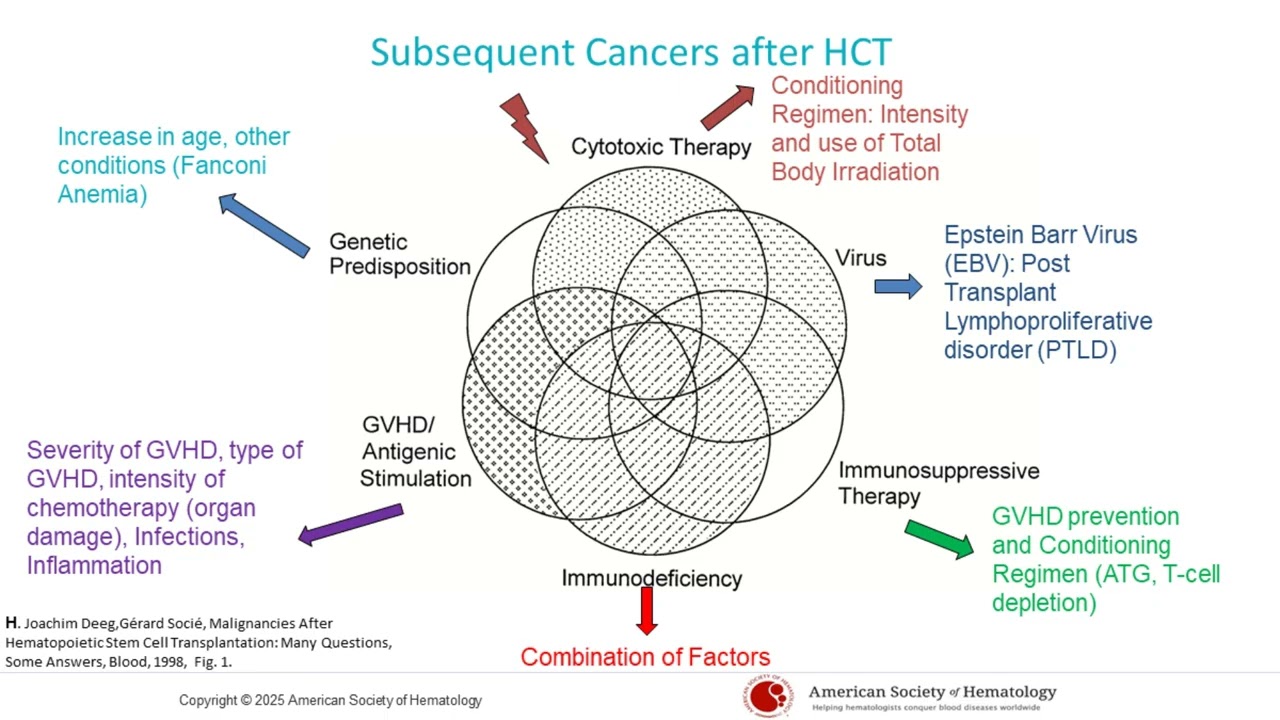
(08:47): Second cancers in the transplant setting can be associated with infection, chemotherapy, and genetic predispositions. So what are the associations, and how do these cancers develop in the setting of transplant? Here is a study that was done in 1998, which is still relevant today. It describes the association of multiple factors such as infection, chemotherapy, and genetic predisposition with new cancers.
(09:12): The intensity of pre-transplant chemotherapy can increase the risk of second cancers and graft-versus-host disease (GVHD). If we go over these one by one, we can define this a little bit more. For example, all the cytotoxic chemotherapy that a patient was exposed to during the course of their treatments has a cumulative effect. The conditioning regimen for a transplant patient may involve a higher or lower intensity cytotoxic therapy. This intensity defines the risk of subsequent malignancies. Also, the utilization of total body radiation as part of the conditioning regimen is an important risk factor for the development of second cancers.
(10:01): A type of lymphoma after transplant, called post-transplant lymphoproliferative disorder (PTLD), may be caused by a prior Epstein-Barr infection and the level of immunosuppression after transplant. An infection from the Epstein-Barr virus, which commonly causes mononucleosis in young adults, may be present in a patient undergoing a transplant. When there is immunosuppression after the transplant, this virus can expand and may cause a type of lymphoma called post-transplant lymphoproliferative disorder, or PTLD.
(10:39): The level of immunosuppression after a bone marrow transplant can increase the risk of developing PTLD, especially after transplants that use T-cell depletion to prevent graft-versus-host disease (GVHD). Immunosuppressive therapy, the type of GVHD prevention used, especially T-cell depletion, the conditioning regimen, and the utilization of ATG or anti-thymocyte globulin can increase the risk of these infections and subsequently increase the risk of developing this malignancy.
(11:42): Immunodeficiency after transplant can reduce the immune system’s ability to detect and fight new cancers. Immunodeficiency after transplant, caused by a number of factors, including the development, type, and intensity of chemotherapy, can cause organ damage and increase the risk of graft-versus-host disease (GVHD). GVHD decreases the ability of the new immune system to detect new malignancies. It's common for patients with severe chronic GVHD to develop skin cancers as a result of that as well.
(11:48): Regarding genetic predisposition, there are other diseases that increase the risk for malignancy, despite the use of transplantation. One of these is Fanconi Anemia. As mentioned before, increasing patient age also increases the risk of other malignancies.
(12:07): The risk of disease relapse is highest soon after transplant. In the course of the transplantation, as you look at this graph, the risk of a disease relapse is really high in the beginning and decreases over time among survivors of the bone marrow transplant.
(12:37): Early on, there is a risk of post-transplant lymphoproliferative disorder (PTLD) when patients are on the highest degree of immune suppression. Also, during this time period, there is a risk of developing myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) as a result of the patient's exposure to cytotoxic therapies. And then, there is an ongoing risk of second cancers that increases over time.
(13:04): The timeframe when patients typically develop a new cancer differs by disease. Some cancers occur over seven or eight years, while others such as myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) occur earlier.
(13:22): In patients transplanted for Fanconi Anemia, the risk of a second cancer is almost 50 times higher than seen in the general population. This slide shows a study in children, and I would like to point out the risk associated with Fanconi anemia. Fanconi anemia is a genetic disorder, that interferes with the genetic repair that happens in our cells. When there are mutations in the cells, patients have a higher risk of developing cancers like head and neck cancers, and they're very susceptible to cytotoxic therapy-related cancers.
(13:53): As you can see with Fanconi Anemia (row is highlighted in blue) you have a second cancer malignancy risk of 5.4% and there is almost a 50 times higher risk of cancer compared to the normal population of the same age.
(14:46): Several studies were done on multiple myeloma to try to understand other factors associated with second malignancies. Both cytotoxic therapy (used as a treatment in myeloma especially auto-transplantation) and other drugs, such as lenalidomide (Revlimid®), also increase the development of malignancies.
(14:49): The magnitude of the risk is shown on this graph. You can see here that the development of cancer is less than 10% over time in this study. This is lower than the other risks, such a recurrence of the cancer or death related to multiple myeloma. This study is relatively old.
(15:28): We have another study that was done, using CIBMTR registry data, looking at this question. It looks at different events over 15 years. The development of secondary cancers is about 10%. The development of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS)is less than 5%. The risk of relapse and mortality related to the multiple myeloma is much higher. This gives you a visual about the magnitude of the events that are expected and the timeline when they may happen.
(16:08): A multiple myeloma study was also done to understand the role of maintenance using a drug called lenalidomide (Revlimid®) over time. Patients were randomized, or divided into two groups, one receiving a placebo and the other receiving lenalidomide (Revlimid®) continuous therapy.
(16:31): There was an increased risk of a new malignancy for patients who received lenalidomide. But the overall benefit of receiving lenalidomide, even with the risk of second malignancy, was that patients experienced longer survival. Still, the fact remains that treating patients with lenalidomide after autotransplant does increase the risk of a second malignancy. This emphasizes the importance of watching for these cancers and educating patients about this risk.
(17:13): Looking on this slide you can see the number of autologous transplants, allogeneic transplants, and CAR T-cell therapies done annually in the U.S. over time. The number of patients who are receiving transplants is increasing. Not only that, there is an increase in survival, and it's estimated there'll be an increasing number of transplant survivors, given the improvement in survival after transplant. So surveillance for these cancers and patient education are important.
(18:03): Overall survival after autologous and allogeneic transplants has improved over the last two decades. On the left of this side you can see allogeneic transplants, while the right shows autologous transplants. This is an overall survival curve, and each of those lines represents a period of time. As you can see, the higher the line is the less the mortality. In more recent years, even for allogeneic transplants, the overall survival of patients is much better than two decades ago. The probability of being a long-term survivor of an allogeneic transplant or an autologous transplant has improved.
(18:40): The risk of developing a second malignancy after transplant is 1% for patients younger than 18, but is 2% for those over 18 years old. So, what is the impact of the second malignancies that happen after a transplant? Here are two pie charts: one for patients under 18 years of age at the time of transplant and the other for those over 18. Unfortunately, the main cause of death after an allogeneic transplant is that the disease returned. The risk of developing a second malignancy after transplant is 1% for patients younger than 18, but is 2% for those over 18 years old.
(19:28): In summary, second cancers can occur after transplant, although the risk is low. Cancer education and prevention are part of all survivorship guidelines for patient care.
(19:41:) The factors that reduce the risk are: (1) improved understanding of the risk; (2) doing frequent screenings for cancers, including colonoscopy and mammograms, skin exams, tests for prostate specific antigens, blood tests, oral health assessment, pap smears, and other methods for preventive health. Following wellness practices such as a good diet, decreased tobacco use, and reducing exposure to UV light that could cause skin cancer.
(20:20): We'll now shift to CAR T cell therapy. CAR T cells are genetically manipulated cells that are infused into the patient to fight the cancer. As with any genetically modified product, the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) require that patients who received these products be followed for 15 years to understand the risk of complications. The most pertinent one is that of developing other cancers from the genetic manipulation of the cell before its infusion into the patient. The genetic manipulation it can disrupt important genes and lead those cells to turn into a cancer.
(21:24): Several CAR T-cells are approved in the United States and are listed in blue here. The first one was approved in 2017 with additional ones that followed. Additionally, what happened after approval was increased utilization of the CAR T-cells in earlier phases of the disease, or in other similar diseases that express the same target as those for which the product was initially tested.
(22:00): The Center for International Blood & Marrow Research (CIMBTR) monitors late effects after CAR T-cell therapy, including new cancers. Since CIBMTR collects information about CAR T-cell procedures, we worked to figure out how to respond to the requirements for long-term follow-up of patients who received CAR T cells.
(22:00): We worked with each of the manufacturers of CAR T-cells and developed a project to follow patients long-term. We do not dictate the care the patient receives but collect information on what happens to them whenever they are seen by their local physician. Patients need to sign an informed consent to share information with the CIBMTR, and we then collect information related to the product, the disease, and the outcomes after CAR T. The main focus is to understand the magnitude of the risk of second malignancies and understand whether CAR T-cells lead to a specific type of cancer that is specific to the CAR T-cell.
(23:14): I showed this slide before. This shows the increased use of CAR T-cells over time. There are a total of 23,000 patients who have received CAR T-cell therapy.
(23:28): CAR T-cells may increase the risk of subsequent cancers related to T-cells and these risks are currently being evaluated. In November of 2023, the FDA communicated concerns over whether the utilization of CAR T-cells to treat patients with myeloma, lymphoma, and acute lymphoblastic leukemia could increase the risk of a specific type of cancer related to T-cells. As you know, T-cells are manipulated to produce CAR T-cells, and this causes a lot of concern about the risk for a new cancer post treatment.
(24:08): A group of investigators came together to try to understand the magnitude of the risk. As mentioned earlier in this presentation, there are inherited risks for any cancer therapy, and the question is whether the risks are outweighed by the benefits of this therapy.
(24:35): The risk of second cancers after CAR T-cell therapy is relatively low (less than 1% to 6.4%) and varies with the type of second cancer. When we looked at the CIBMTR database, we looked at the total number of patients who underwent CAR T-cell therapy for different indications as seen in this table. We saw that a total of 6.4% of patients who had non-Hodgkin lymphoma, and 6.4% patients who had multiple myeloma developed a new cancer. It was interesting to note that only 1.6% of patients with acute lymphoblastic leukemia (ALL), developed acute myeloid leukemia (AML) which was reported as second malignancy.
(25:19): On further analysis, we learned that some patients (depending on their type of acute lymphoblastic leukemia) after exposure to CAR T-cells may relapse with a different type of leukemia called acute myeloid leukemia (AML). This is called lineage switch and is not necessarily a new cancer related to CAR T-cell therapy. If we were to remove these seven cases, the risk of subsequent malignancy in children after CAR T-cell ends up being less than 1%.
(25:48): You can see the variability between 6.4% and less than 1% depending on indication. There is a significant difference in age between patients with non-Hodgkin lymphoma and those with myeloma for whom the medium age is over 65. Most patients who have acute lymphoblastic leukemia (ALL) are mostly children.
(26:11): The most common malignancies reported to CIBMTR after CAR T-cell therapy were skin malignancies and myelodysplastic syndrome (MDS). We identified only a handful of cases considered T-cell malignancies, but we could not, based on information shared with us, establish a causality between the CAR T-cell manufacturing and the development of these T-cell malignancies. All of those listed on the slide, even though they have different names, are all different kinds of T-cell lymphoma.
(26:50): A study was done in France by the French registry looked at the risk for developing of T-cell cancers that could be related to the creation of CAR T-cells. Of 3,000 patients, there were no observed cases of these T-cell malignancies. This shows that it is a rare event.
(27:15): There are some cases in the literature, such as a patient who received CAR T-cells to treat his myeloma. He subsequently developed a T-cell malignancy in the gastrointestinal tract where markers for CAR T-cells were detected, suggesting there could be an association with CAR T-cells and this cancer. However, these reports are very rare and the issue is still being studied.
(27:49): Hence, if we translate the information that we learned from transplant to the CAR T-cells, we can say the impact of cytotoxic therapy on the development of second cancers is similar. But if CAR T-cell patients received lymphodepleting therapy, bridging therapy, or any other therapy prior to CAR T-cell therapy, that may increase the risk of second malignancies.
(28:12): Similarly, infections can happen because CAR T-cell patients become really immunosuppressed, and may develop second malignancies as well, similar to those seen after transplant.
(28:24): The CAR T-cell type is important because manufacturing is different for each type of CAR T-cell. Any additional treatment after CAR T-cell therapy may increase the chance for subsequent malignancies as well.
(28:45): There is an ongoing immune dysregulation after CAR T-cells, because the part of the immune system responsible for production of antibodies is extremely inhibited. CAR T-cell complications due to increased inflammation may have an effect on development of myelodysplastic syndrome (MDS). Older age, or any prior cancers, may also have the same effect.
(29:15): In summary, the risk of second cancers after CAR T-cell therapy is present and differs by disease. As I mentioned earlier, the risk for lymphoma and myeloma patients is close to 6%. The risk for patients with acute leukemia is less than 1%. This difference could be the effect of the patient's age and the therapy that they received prior to the CAR T-cell.
(29:37): The most common cancers seen after CAR Tare skin cancers and myelodysplastic syndrome (MDS). MDS appears to occur a little earlier than what is observed after transplant, which is a point of study at the moment.
(29:49): Active cancer screening and annual visits can reduce the risk of second cancers after CAR T-cell therapy. What can be done to reduce the risk? The answer is active cancer screening (similar to what is recommended for transplant patients) and annual visits over 15 years will help understand if other issues lead to the development of second malignancies.
(30:11): The benefits of CAR T-cell therapy outweigh the risks. In conclusion, second cancers are an unfortunate complication of CAR T-cell therapy and transplants. While the risk is low the benefit from these therapies outweighs the risk. The risk increases with age and other factors as mentioned earlier.
(30:27): Several strategies may be used to reduce the risk of second cancers after CAR T-cell therapy. Understanding who is at risk increases our ability to monitor and treat patients earlier. Mitigating the inflammatory complications of the CAR T-cells, limiting the intensity of the regimens used for transplant, and reducing the risk of graft-versus-host disease may reduce the risk of second cancer. I'll be happy to take questions.
(30:57): Steve Buechler: Q & A. Thank you Dr. Pasquini for this excellent presentation. We'll now begin the question and answer session. Our first question is - After autotransplant, would CAR T possibly be used to treat a secondary cancer?
(31:21): Dr. Marcelo Pasquini: Yes. The indications for CAR T cell therapy do not change. It depends on what the malignancy is after transplant. If, after transplant, acute lymphoblastic leukemia develops, CAR T-cell therapy can be used for treatment. So it's possible.
(31:41): At the moment CAR T-cells are limited to those cancers that express a certain target on their surface. For now, there are two types of targets that CAR T-cells are used against. One is called CD19, which is expressed in lymphoma and acute lymphoblastic leukemia, and the other is BCMA or B-cell maturation antigen, which is commonly expressed in patients who have multiple myeloma.
(32:12): Steve Buechler: Thank you very much. This person wants to know - What is a common disease trajectory post-CAR T when the diagnosis was diffuse large B-cell lymphoma (DLBCL) in the central nervous system (CNS)?
(32:38): Dr. Marcelo Pasquini: Right now there is limitation on using CAR T-cells for this indication. Looking at the registry, there is an increased interest for patients who have central nervous system lymphoma, which means involvement of the cancer in the brain and the fluid that surrounds the brain. That can happen in two different instances. Either there's primary CNS lymphoma, which means the cancer is only in the central nervous system and nowhere else, or the cancer originated in a tissue and also expressed in the brain.
(33:32): In essence, the trajectory after the treatment for these conditions is slightly different than for patients without involvement of the CNS. Patients with CNS do respond to CAR T-cell therapy, but the response rate is not as high as that of patients who do not have CNS lymphoma. So, the indication is to proceed with CAR T-cells, because there is circulation of CAR T-cells in the central nervous system, which allows them to have activity in the tumor as well. I hope I answered the question.
(34:14): Steve Buechler: Thank you for that. Here's an either/or question. “Are secondary cancers caused by the chemo infused during transplant, or because the body reacts to getting its stem cells back”?
(34:26): Dr. Marcelo Pasquini: That's a good question. The classical chemotherapies that affect the cell division are toxic to the DNA. So the cells and organs that are most sensitive to the effects of the chemo are organs in tissues that are constantly undergoing cell division.
(34:54): Your bone marrow, for example, constantly produces blood. Thus, whenever chemotherapy is present, it can cause some changes in the DNA of these cells in the bone marrow, which increases the risk of developing myelodysplastic syndrome (MDS). The effect is not necessarily related to stem cells but related to the chemotherapy that is utilized.
(35:24): Historically, depending on the type of chemotherapy, there's what we call latency, or expected time that a new cancer may occur. Some types of chemotherapy may take about five years, and others about seven years. That's well described in literature.
(35:40): What is being studied right now is whether the chronic inflammation that happens with complications after transplant, or complications of CAR T-cell therapy, plays a role in increasing the risk for second malignancies that may occur with that overstimulation of the bone marrow. In short, the association is mostly related to the chemotherapy.
(36:11): Steve Buechler): Thank you. This person wants to know - "Can you elaborate on the Ide-cell (Abecma®) CAR T treatment? Can I receive this treatment if I have already received Abecma®, which has run its course? Are there any treatments that would prevent the use of Ide-cell?"
(36:28): Dr. Marcelo Pasquini: That's a question related to use of CAR T-cells after CAR T-cells. What is important to know is that the CAR T-cells, especially for myeloma, are focused on a single target. I predict that in the future we'll have additional CAR T-cells that will actually affect more than one target and allow us to have a little better control. The question is if you go from one CAR T-cell that goes to a specific target (BCMA) and then there is another available CAR T-cell that goes to another target (GPRC5D), do you have a chance to have better control?
(37:17): The answer theoretically is, yes. The number of patients who have gone from one to the other is still low. We can extrapolate from the utilization of what we call bispecific antibodies, which go to different targets, and alternating the target helps us have some improvement on disease control. If you have two agents, either CAR T-cell or an antibody that goes to the same target, the second one decreases the effect that you had from the prior treatment. The idea right now for treatment of myeloma is to try go to a different target.
(38:08): So the answer to the question is yes. Going to another CAR T-cell product that has a different target may increase the chance for disease control.
(38:20): Steve Buechler: This person starts by thanking you for your talk, and they refer to the slide that demonstrated the causes of death for patients post-allogeneic stem cell transplant. Apparently it said that six percent GVHD, and she wants to know, is that usually from acute GVHD?
She says, I think of the cause of mortality from chronic GVHD is associated with infection and organ failure, but in this particular graph it specifies GVHD as a factor different than infection and organ failure."
(38:49): Dr. Marcelo Pasquini: That's a very good point. Thank you for raising this. This data comes from the registry, and the causes of death are what the centers tells us. There's no review of exactly what the actual cause is.
(39:10): We try to understand what the root cause was. In the registry we ask about the primary cause of death, and at least up to five contributory causes of death, because things can be complicated, and you can have multiple causes. As you noted correctly, you could have had GVHD that caused the risk for infection, and that was ultimately the cause of infection. But sometimes GVHD was the root cause of it.
(39:41): You're right that GVHD, as a cause of death, is usually associated with either organ failure or infection, not the GVHD. Or it is related to the treatment of the GVHD.
(39:57): I think it's important to note that we take what the center is telling us at face value, and if the center says that the primary cause was GVHD and the secondary cause was infection, then that's why shows up that way that graph. When you have infection and organ failure as the cause of death, those both could be related to GVHD. Whether it is acute, or chronic, depends on the timing. You could have severe acute GVHD that can lead to these complications, or chronic GVHD. We don't separate the two types on the graph.
(40:45): Steve Buechler: Thank you for that. The next person reports, "I now have myasthenia gravis. After a clinical trial for GPRC5D CAR T therapy, can I have another CAR T to get rid of the myasthenia?
(41:01): Dr. Marcelo Pasquini: That's a good question. This is a question about using CAR-T cell therapy for treatment of autoimmune disorders. There is some emerging data, but not a lot of experience with that.
(41:30): There is a clinical trial looking at this specifically. I cannot tell you what the likelihood of benefit is for treating this complication with CAR Ts. But I understand that this is now going to be a niche, using CAR T-cells for autoimmune diseases.
(41:53): Most commonly the indications are multiple sclerosis, myasthenia gravis, lupus erythematosus and lupus nephritis, and perhaps scleroderma or systemic sclerosis. These are the five different indications. There are clinical trials on now trying to understand whether using CD19-targeting CAR T-cell will increase the chance of controlling autoimmune disease.
(42:22): Steve Buechler: Thank you for that. The next person wants to ask - What do you think about looking at myeloid genes panel pre-CAR T, and what would you do with the information?
(42:33) Dr. Marcelo Pasquini: We don't know. This is a very good question. We do know that as you get older, you accumulate some of these mutations, and they are small clones that we can detect in the bone marrow. As you develop them over time, that can increase the risk for development of myelodysplastic syndrome. These panels will detect those clones.
(43:12): The problem is that clinically, we don't know what to do with them, aside from monitoring. Some of these clones that appear in these panels may regress over time. It's not a direct linear fashion that people who have it will have increased risk.
(43:31): There is an increased risk, but the risk is not fatal. You cannot just say yes, it's a hundred percent or 90%. We don't know what to do. There are a lot of studies looking at this right now, looking at both - before the CAR T-cells and also over time. This is to see whether these clones expand or decrease over time. It is still a point of scientific exploration to understand what it is. Clinically, doing this or not, would not really impact the decision to move to a CAR T-cell. This might change in the future, but at the moment this still remains investigational.
(44:18): Steve Buechler: Does GVHD itself leave you more open to secondary cancers, or is it the steroid treatment that's causing a higher risk?
(44:27): Dr. Marcelo Pasquini: That's a very good question. An immune system, as you know, is a very complex mechanism. The immune system utilizes surveillance to see whether a cancer is developing. Throughout our lives, we may have injuries to our DNA, and our immune system takes care of that. For you to develop a malignancy, that cancer has to evade that initial surveillance mechanism.
(45:09): Having said that, after a transplant, patients with an ongoing graft-versus-host disease that’s difficult to control (mostly chronic GVHD), tend to have decreased immune surveillance. So that's why I included in one of those graphs that immune suppression, or immune deficiency, either caused by the graft-versus-host disease or the treatment of the GVHD may increase the risk of a new cancer.
(45:45): We see a lot of association with skin malignancies because increased exposure to UV rays can elicit breaks in the DNA, and that can lead to basal cell cancer or squamous cell cancer. It is interesting that you have the same issue with some cancers.
(46:15): For example, in chronic lymphocytic leukemia is associated with other cancers. The association of chronic lymphocytic leukemia and prostate cancer is well known, or chronic lymphocytic leukemia and skin cancer are very well known, regardless of whether the patient receives a CAR T-cell or a transplant. It has to do with your immune system's ability to recognize some of these elements that are appearing.
(46:43): Steve Buechler: This may be our shortest question of the day. How often do you recommend colonoscopies?
(46:48): Dr. Marcelo Pasquini: That's a good question. I usually follow the recommendations for preventive health, which are related to age and family risk. It's possible that patients may have a sibling, or a parent, who had colon cancer at a younger age. This decreases the age at which you start the colonoscopy.
(47:23): There are two tests that look at DNA for surveillance. You may start these as early as age 40, and then repeat them every five to ten years, depending on whether any abnormalities were seen, and your family history. That also plays a role. It has to be individualized for the patient on when to start.
(47:55): Steve Buechler: Next question is – At what point will the secondary cancer risk diminish?
(48:13): Dr. Marcelo Pasquini: Let's say you never had cancer before, just the fact of being alive increases your risk of having cancer, because you're aging. That is really the baseline of the question. I think that we know the expected risk of aging.
(48:34): Certain cancers may occur earlier than others, as I showed in one of the slides. So, beyond that point, for example, if you are two years out after your allogeneic transplant, and you're not on immune suppression, and you have no active GVHD, your risk for post-transplant lymphoproliferative disorder (PTLD) is very low. It will decrease over time as you regain your immune system.
(49:06): However, anybody who receives an auto or allogeneic transplant or any cancer therapy has a higher, cumulative risk of a second malignancy compared to the people who never had cancer or these treatments. So, the risk will increase with age, but certain kinds will decrease as soon as you recuperate from the transplant.
(49:35): Steve Buechler: The next person wants to know – Do intravenous immunoglobulin (IVIG) infusions increase your risk for a second cancer?
(49:43): Dr. Marcelo Pasquini: There's no association between the two. Immunoglobulin replacement is a preventive measure for infections. Receiving CAR T-cells will have suppressed the normal cells that produce antibodies. So they are not able to fight infection, especially upper respiratory infections. This happens very frequently.
(50:10): More often than not, we recommend that these patients have Immunoglobulin replacement if they have repetitive infections, or if they have a very low number of antibodies (which is measured by quantitative immunoglobulin G or IgG levels). The lower the number, the higher the risk of infection. No studies have reported that IVIG may increases the risk of second cancers.
(50:36): Steve Buechler: Here's an interesting one. If your stem cell donor has a family history of cancer, does that increase your risk of a new cancer after transplant?
(50:46): Dr. Marcelo Pasquini: There are reports that cancer can be donor derived. If you have a sex mismatch transplant, like a woman to a man, or a man to a woman, the cancer may be caused by the donor XX or XY chromosome.
(51:12): Now that depends on the type of the cancer. If the donor had a had a history of colon cancer, it's unlikely that that will transmit into the recipient, because the problem is mostly in the organ, in the colon, not necessarily in the bone marrow. The cancers that have been reported to be transmitted from the donor to recipient are usually of cancers that affect the bone marrow origin, for example, leukemia or a lymphoma that you're able to detect. There are cases that we've seen with that.
(51:56): Steve Buechler: Thank you for that. We're going to have to make this the last question. Can you tell us what this CIBMTR data is, and how far back it goes? And what is the role of that data?
(52:15): Dr. Marcelo Pasquini: The CIBMTR is Center for International Blood and Marrow Transplant Research. It was established in Milwaukee in the 1970s to understand outcomes after transplant. It was one of the first outcomes databases that were created. It's funded by the National Institutes of Health (NIH).
(52:48): We have a contract with every center in the United States that does bone marrow transplants, and we collect information on all patients treated at that center who sign a consent form. If you had a transplant, you may remember that someone told you about the data registry on the CIBMTR. The patient’s consent is to share information that is de-identified about the outcomes of the transplant. It is a unique resource for the transplant community. We use that to do numerous studies, to conduct clinical trials ,and help design clinical trials.
(53:30): Since 2007, there has been a mandatory report required by the U.S. Department of Health and Human Services (HHS) about all allogeneic transplants. The data collected is used to understand the quality of outcomes for patients.
(53:49): The data we collect from transplant centers is utilized to run what's called the center-specific analyses that benchmarks the centers based on the patients that are treated. Centers are evaluated, based on the expected survival of the patients that they treat. Their outcomes can be rated as expected, better than expected, or worse than expected. That’s become a report card for centers. It improves the care of the patients.
(54:17): The data is also utilized for research and for quality assessments. Also the manner in which the centers collect their data is reviewed by the Foundation for Accreditation of Cellular Therapy (FACT) to accredit the centers. So the CIBMTR is a resource, and the data is used mostly for research, but also for these other purposes. Since 2017, we've been using the CIBMTR to collect CAR T-cell outcomes to understand the outcomes of second malignancies.
(54:48): Steve Buechler: Closing. Lots of good questions and lots of good answers. That's going to have to do it. On behalf of BMT InfoNet and our partners, thank you Dr. Pasquini for a very helpful presentation, thank you the audience for your excellent questions. Please contact BMT InfoNet if we can help you in any way.